How Do Enzymes Affect Chemical Reactions Explain
Enzymes create chemical reactions in the body. They actually speed up the rate of a chemical reaction to help support life.

Enzyme Rate Of Reaction Factors Catalysts Video Lesson Transcript Study Com
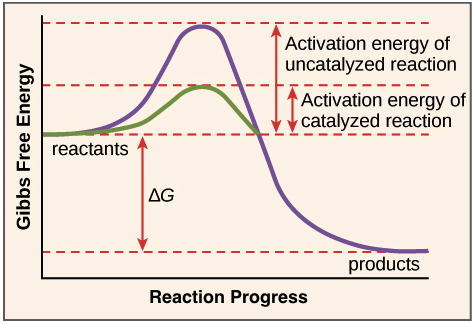
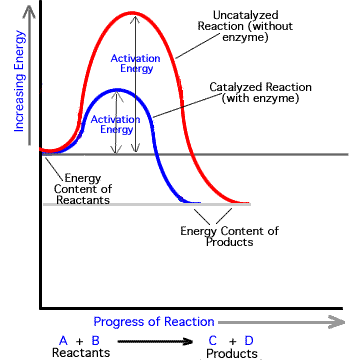
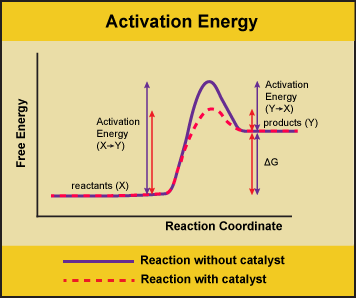
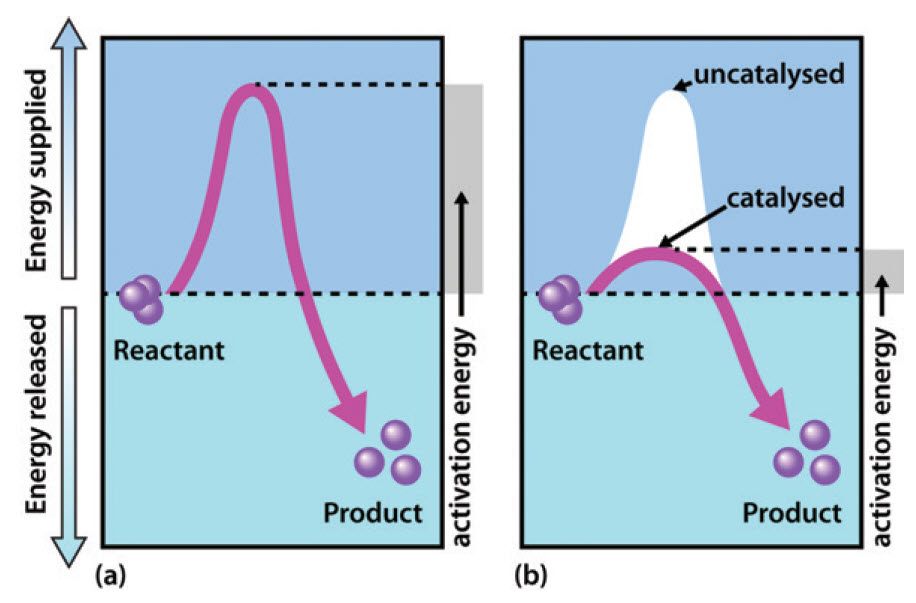
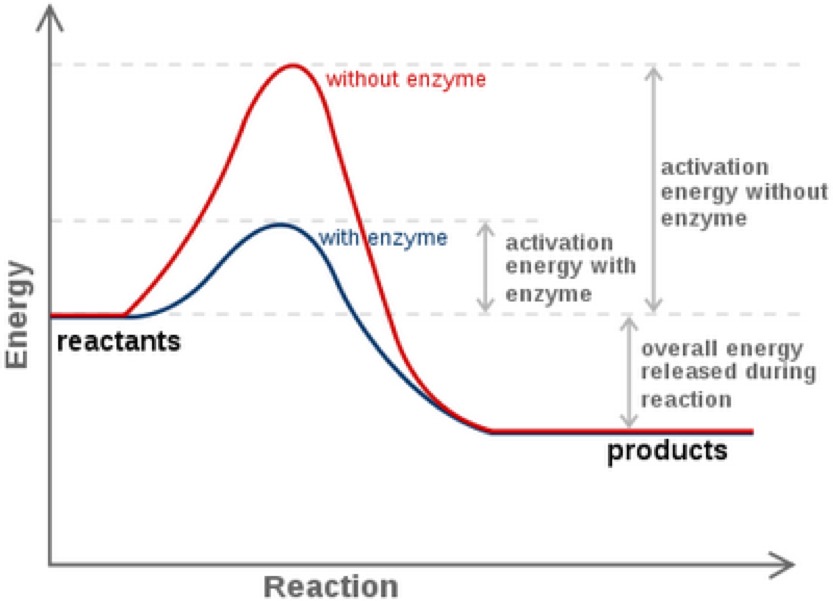
The enzyme speeds up the reaction by lowering the activation energy needed for the reaction to start.
How do enzymes affect chemical reactions explain. Investigating the Effect of Temperature on Enzyme Activity Almost all chemical reactions that occur in living organisms are catalyzed by enzymes. Compare the activation energy with and without the enzyme. Competitive and Noncompetitive Inhibition.
See full answer below. Features of Enzyme Catalyzed Reactions Enzymes are biological catalysts. An enzyme functions by lowering the activation energy of a chemical reaction inside the cell.
Enzymes are known as catalysts in a chemical reaction as they can reduce the time it takes for the reaction to occur by breaking things down. Become a member and. Become a member and.
Thus enzymes speed up reactions by lowering activation energy. Enzymes are important to living things because they can be found throughout the body to aid in things like digestion and fighting illnesses. Activation energy is the energy needed to start a chemical reaction.
In general the lower amount of activation energy that a potential reaction has the faster the rate of reaction will be. Enzymes speed up catalyze chemical reactions. In enzyme-catalyzed reactions the enzymes lower the activation energy needed for a certain chemical reaction.
Enzymes are critical in all metabolic reactions because they help to speed up the reactions but still allow the living thing to maintain a constant. Enzymes lower the activation energy by binding to the reactant molecules and holding them in such a way as to speed up the reaction. The free energy of the reactants and products do not change just the threshold energy level needed for the reaction to commence.
Enzymes lower the activation energies of chemical reactions. Enzymes therefore allow scientists to control the exchange of atoms mechanically as explained by Science Daily. Enzymes speed up chemical reactions by lowering the amount of activation energy needed for the reaction to happen.
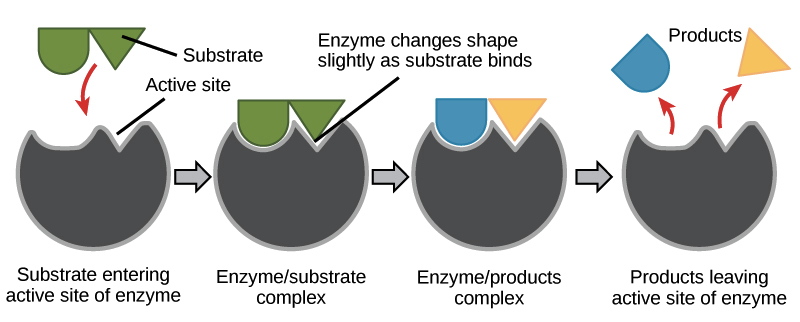
The chemical reactions that keep us alive our metabolism rely on the work that enzymes carry out. A process that changes or transforms one set of compounds into another. Enzymes change shape during the reaction process which allows them to efficiently reduce activation rates.
Enzymes catalyze chemical reactions by first binding to molecules and then lining them up in ways that increase the probability of the molecules exchanging atoms when they collide. Many enzymes change shape when substrates bind. Enzymes will usually speed up a chemical reaction and will additionally facilitate it when it would otherwise not occur.
Enzymes can lower the activation energy of a chemical reaction in three ways. A catalyst is a substance that increases the rate at which a reaction occurs. Like all catalysts enzymes work by lowering the activation energy of chemical reactions.
Explain how enzymes cause chemical reactions to occur more readily. They are secreted along the digestive tract. Enzymes speed up chemical reactions by lowering activation rates.
Catalysts lower the activation energy for reactions. Enzymes which are proteins behave as catalysts in chemical reactions. The enzymes of the digestive tract include pepsin trypsin and peptidases which break down proteins into amino acids.
They work by lowering the activation energy making it easier for chemical reactions to begin. Activation energy is the energy needed to form or break chemical bonds and convert reactants to products Figure 4. Many factors in a cells environment affect the action of an enzyme.
The enzyme speeds up the reaction by lowering the activation energy needed for the reaction to start. The enzymes in your body help to. In some cases enzymes can make a chemical.
In this investigation you will design an experiment to determine the effect of temperature on an enzyme-catalyzed reaction. You will complete an. In cells they promote those reactions that are specific to the cells function.
See full answer below. The lower the activation energy for a reaction the faster the rate. Enzymes generally lower activation energy by reducing the energy needed for reactants to come together and react.
Compare the activation energy with and without the enzyme. Because enzymes ultimately determine which chemical reactions a cell can carry out and the rate at which they can proceed they are key to cell functionality. Digestive enzymes are involved in chemical reactions that break down the food we eat and convert them into energy.

Enzymes In Biochemical Reactions Role Importance Expii

How Do Enzymes Speed Up Reactions Quora
3 10 Chemical Reactions In Living Things Human Biology
How Do Enzymes Speed Up Reactions Quora
Enzyme Structure And Function Article Khan Academy

Enzymes Biology For Non Majors I
Enzymes And The Active Site Article Khan Academy
Enzymes And The Active Site Article Khan Academy
How Do Enzymes Speed Up The Chemical Reactions Use Activation Energy In Your Answer Socratic
Chemical Reactions Opencurriculum
5 2 Enzymes Biology Libretexts
Chapter 6 Enzyme Principles And Biotechnological Applications Chemistry
Http Www 3dmoleculardesigns Com 3dmd Files Enzyme In Action Pdfs Enzymeteacherskey Forweb1 Pdf
How Enzymes Speed Up The Chemical Reactions Ck 12 Foundation




Post a Comment for "How Do Enzymes Affect Chemical Reactions Explain"